Presented by Zia H Shah MD
Ancient Viruses Built Your Brain and Body
Audio teaser:
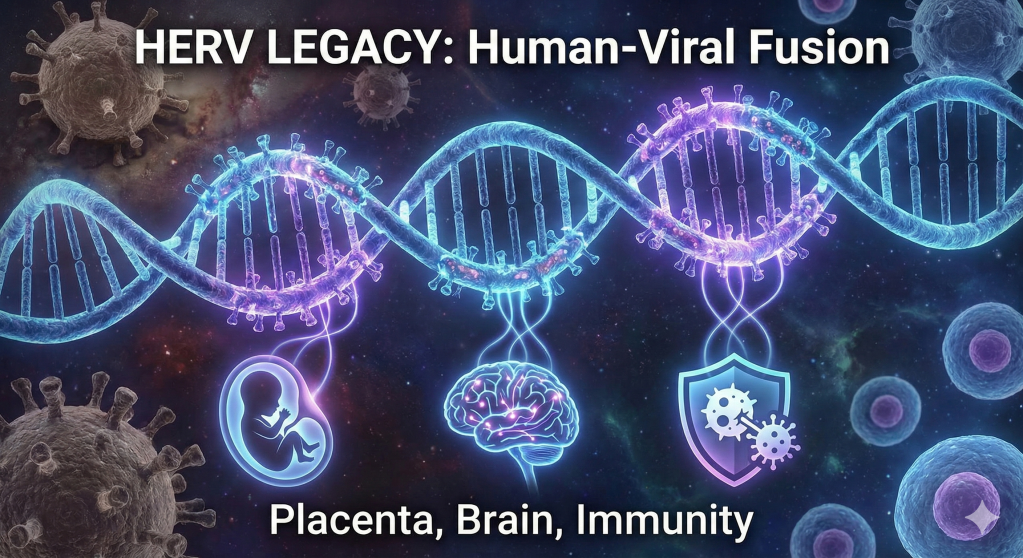
The traditional view of the human genome as a stable, purely mammalian sequence of instructions has been fundamentally dismantled by the emergence of paleovirology and high-resolution genomic mapping. Biological reality suggests that the human identity is not a solitary lineage but a complex, chimeric assembly. Approximately 8% of the human genetic code is composed of ancient viral DNA, remnants of ancestral infections that occurred millions of years ago. These sequences, known as human endogenous retroviruses (HERVs), represent the fossilized traces of exogenous retroviruses that successfully integrated into the germline of our ancestors, ensuring their vertical transmission across countless generations. Far from being inert “junk DNA,” these elements have been “usefully employed” for the most fundamental processes of human existence, including the development of the placenta, the regulation of the brain’s cognitive architecture, and the defense against modern pathogens. The transition of these elements from lethal parasites to essential symbiotic partners represents one of the most profound narratives in evolutionary history, effectively blurring the boundary between the host and the viral “other”.
The human genome is a palimpsest, a record of ancient battles and unlikely alliances where the distinction between “self” and “foreign” has been erased by the slow grind of evolutionary time. We are, in a very real biological sense, descendants of viruses as much as we are descendants of primates. The 100,000 fragments of retroviral DNA embedded in our chromosomes are not mere historical debris; they are active, vibrant participants in the daily business of life. They serve as the engineers of our embryos, the sentinels of our immune systems, and the architects of our memories. This integrated genomic ecosystem challenges our philosophical understanding of individuality, suggesting that the “human” is a collaborative masterpiece built upon the captured genetic hardware of ancient pathogens. As we delve into the thousands of active HERV loci, we uncover a story not of infection and decay, but of transformation and transcendence—a legacy where the ghosts of ancient viruses provide the vital spark for modern human existence.
The Genomic Landscape: Origin and Architecture of Human Endogenous Retroviruses
The presence of human endogenous retroviruses is the result of a process known as endogenization. This occurs when an exogenous retrovirus infects a germline cell—a sperm or an egg—and its proviral DNA is successfully integrated into the host’s chromosomal DNA. If this infected cell contributes to a successful fertilization event, the viral sequence is then present in every cell of the offspring and is inherited by subsequent generations in a Mendelian fashion. Over millions of years, these sequences have undergone repeated rounds of amplification and transposition, leading to their widespread distribution throughout the genome.
Taxonomic Diversity and Classification Hierarchies
The human genome contains approximately 100,000 fragments from various ancestral retroviruses, categorized into roughly 30 to 40 distinct families based on their phylogenetic relationship to modern exogenous retroviruses. These families are primarily grouped into three major classes, each reflecting a different ancestral lineage and period of integration.
Class I HERVs are characterized by their sequence similarity to Gammaretroviruses, such as the Murine Leukemia Virus (MLV), and Epsilonretroviruses. This class represents some of the most ancient and abundant insertions, including the widely studied HERV-H and HERV-W families. Class II HERVs show homology to Betaretroviruses, specifically the Mouse Mammary Tumor Virus (MMTV). The HERV-K superfamily, which is considered the most biologically active group in modern humans, belongs to this class. Class III elements are related to Spumaviruses, or foamy viruses, and are exemplified by the HERV-L family, which plays a critical role in the very first divisions of the human embryo.
The table below delineates the characteristics of several representative HERV families identified in high-resolution studies, highlighting their estimated ages and structural attributes.
| HERV Family | Primary Location (Chromosome; Band) | tRNA Primer | Estimated Age (106 years) | Copy Number in Genome |
| HERV 1 | 10; q11.21 | Pro | 12.2 – 29.7 | ~21 |
| HERV 2 | 1; p31.1 | ND | 49.5 | ~8 |
| HERV 4 | 4; q13.3 | Glu | 24.2 – 62.2 | ~170 |
| HERV 5 | 3; p25.1 | Gln | 25.9 – 41.2 | ~27 |
| HERV 10 | 6; q22.31 | ND | 18.0 – 33.0 | ~65 |
| HERV 11 | 1; p35.2 | ND | 19.3 – 30.1 | ~67 |
| HERV 12 | 3; q26.1 | ND | 24.9 – 25.9 | ~33 |
| HERV-K (HML-2) | Multiple | Lys (K) | 0.1 – 5.0 | ~91 proviruses |
| HERV-H | Multiple | His (H) | ~30.0 | ~1,000 elements |
Structural Components of the Proviral Genome
An intact HERV provirus mimics the genomic organization of modern exogenous retroviruses like HIV. It is composed of four primary open reading frames (ORFs): gag (encoding structural proteins like matrix, capsid, and nucleocapsid), pro (encoding viral protease), pol (encoding reverse transcriptase, integrase, and RNase H), and env (encoding the envelope glycoprotein responsible for receptor binding and membrane fusion). These coding sequences are sandwiched between two long terminal repeats (LTRs).
The LTRs are particularly significant from a regulatory perspective. They contain the promoters, enhancers, and polyadenylation signals that allow the host cell’s machinery to transcribe the viral genes. Over evolutionary time, many HERVs have undergone homologous recombination between their two LTRs, resulting in the deletion of the internal coding genes and leaving behind a “solo LTR”. There are hundreds of thousands of these solo LTRs scattered throughout the human genome, many of which have been co-opted as alternative promoters or enhancers for human genes. While most of the 100,000 fragments are inert due to the accumulation of mutations and stop codons, several families, particularly HERV-K and HERV-W, retain nearly intact sequences and are capable of producing functional proteins under specific conditions.
Developmental Catalyst: HERVs in the Early Life Cycle
The most dramatic evidence for the functional integration of HERVs into human life is found during embryonic development. Rather than being silenced throughout the lifecycle, specific retroviral elements are transiently and robustly activated to drive key developmental transitions.
Zygotic Genome Activation and the HERV-L Regulatory Axis
The journey of human life begins with a single fertilized cell that must rapidly transition into a multicellular embryo. This process, known as zygotic genome activation (ZGA), requires a massive wave of transcription to replace maternal RNAs with the embryo’s own genetic instructions. Recent research has demonstrated that HERV-L elements (Class III) are indispensable for this transition. In the human embryo, the transcription factor DUX4 binds to the LTRs of repetitive HERV-L elements at the 2-cell stage, initiating a cascade of gene activation necessary for the embryo to progress. Without this viral-driven regulatory network, the embryo cannot successfully transition to a blastocyst, illustrating that retroviral remnants are essential for the very first divisions of life.
Pluripotency and the HERV-H Transcription Network
As development proceeds, the maintenance of stem cell pluripotency—the ability to differentiate into any cell type—becomes paramount. The HERV-H family is uniquely characterized by its exceptionally high expression in human embryonic stem cells (ESCs) and induced pluripotent stem (iPS) cells. In these cells, HERV-H elements produce long noncoding RNAs (lncRNAs) that function as regulatory scaffolds, recruiting coactivators to the promoters of core pluripotency genes like OCT4, SOX2, and NANOG. Experimental knockdown of HERV-H transcripts leads to the immediate downregulation of these genes and a loss of stemness, proving that HERV-H is a central pillar of the human pluripotency network. Furthermore, HERV-H elements help establish the three-dimensional architecture of the genome by defining topologically associating domains (TADs) in a manner dependent on their active transcription.
Placentation: The Syncytin Convergence and Maternal-Fetal Tolerance
The evolution of the placenta is perhaps the most celebrated instance of viral exaptation. The placenta’s function relies on the syncytiotrophoblast, a multinucleated layer that facilitates the exchange of nutrients and oxygen between the mother and the fetus. This layer is formed through the fusion of individual trophoblast cells, a process mediated by two captured viral envelope proteins: Syncytin-1 (encoded by ERVW-1 of the HERV-W family) and Syncytin-2 (encoded by ERVFRD-1 of the HERV-FRD family).
The table below summarizes the roles of these key developmental HERVs.
| HERV Element | Gene Source | Primary Function | Significance |
| Syncytin-1 | env (HERV-W) | Cell-cell fusion (Syncytiotrophoblast) | Essential for placental structure and maternal-fetal exchange |
| Syncytin-2 | env (HERV-FRD) | Trophoblast fusion & Immunosuppression | Prevents maternal immune rejection of the fetus |
| HERV-H | LTR/lncRNA | Stem cell scaffolding | Maintains pluripotency in ESCs and iPS cells |
| HERV-L | LTR | Zygotic Genome Activation | Drives the 2-cell to blastocyst transition |
The co-option of these viral envelope proteins—originally used by retroviruses to fuse with host cell membranes—allows for a seamless placental barrier. Beyond structural fusion, Syncytin-2 provides critical immune tolerance, utilizing the immunosuppressive domains inherent to retroviral proteins to protect the “foreign” fetal tissue from the maternal immune system. This represents a remarkable evolutionary strategy: using the tools of an invader to ensure the survival of the offspring.
The Cognitive Virus: HERVs in Neurobiology and Human Thought
The integration of retroviral elements into the human brain is equally profound, influencing both the molecular mechanisms of memory and the spatiotemporal transcriptomics of the healthy central nervous system.
The Arc Paradigm: Viral Capsids as Information Vectors
One of the most striking discoveries in modern neuroscience is the origin and function of the Arc (Activity-regulated cytoskeleton-associated) gene. Arc is essential for long-term information storage and is required for the plastic changes in synaptic strength that underlie learning. Evolutionary analysis has revealed that Arc is derived from a vertebrate lineage of Ty3/gypsy retrotransposons, which are ancestral to modern retroviruses.
Crucially, the Arc protein has retained its ancestral retroviral behavior. Upon neuronal stimulation, Arc protein self-assembles into hollow capsids that resemble viral particles. These capsids encapsulate Arc mRNA and are released from neurons in extracellular vesicles, traveling across the synaptic cleft to be taken up by neighboring cells. Once inside the recipient neuron, the transferred Arc mRNA is translated to facilitate the removal of AMPA receptors from the synapse, thereby modulating synaptic strength. This “virus-like” intercellular signaling suggests that human cognition is powered by repurposed viral hardware, utilizing a form of domesticated infection to communicate between neurons.
Spatiotemporal Transcriptomics of the Healthy Brain
The transcriptional activity of HERVs in the brain is not limited to developmental stages. Mapping studies have identified thousands of HERV RNAs transcribed in the healthy adult brain, with specific families showing distinct expression patterns across different subregions. Analysis of the HERV-K (HML-2) family has shown that out of 99 relatively intact proviruses, 58 are expressed in the brains of healthy individuals.
Research utilizing digital cytometry and RNA sequencing across 13 brain regions has found that the expression of six specific HERV-K proviruses (located at loci such as 5q33.2, 7q11.21, and 12q24.33) correlates significantly with the presence of brain-infiltrating immune cells, including CD8+ T cells and follicular helper T cells. This implies that HERV-K expression is part of the normal physiological landscape of the brain, potentially acting as a local immunomodulator to maintain homeostasis. Even in a state of health, these “ghosts” of ancient viruses are actively communicating with the host’s immune surveillance system.
Immunological Sentinels: Viral Mimicry and Host Defense
The co-evolution of humans and HERVs has resulted in the “domestication” of viral activities to enhance host immunity. Retroviral elements serve as both structural components of our immune networks and as direct inhibitors of modern exogenous pathogens.
Interferon System Integration and the MER41 Enhancers
The interferon (IFN) response is a critical component of the innate immune system, triggering the expression of hundreds of genes to combat viral infection. Thousands of ancient retroviral sequences have been co-opted as regulatory elements within this system. Specifically, the MER41 family of HERV-derived LTRs contains binding sites for the transcription factor STAT1. When a cell is stimulated by interferon, these MER41 sequences act as enhancers to boost the expression of adjacent antiviral genes, such as AIM2 (which triggers the inflammasome). By dispersing these inducible promoters throughout the genome, HERVs have allowed the human immune system to expand and refine its defensive toolkit, effectively coordinating a complex response across the entire genome.
Retroviral Interference and Super-infection Resistance
Domesticated viral proteins also provide direct defense against modern retroviruses through a mechanism known as super-infection resistance. Some HERV-derived envelope proteins can bind to and occupy the cellular receptors that modern exogenous retroviruses would otherwise use to gain entry into the cell. Furthermore, HERV-K Gag proteins can co-assemble with the Gag proteins of infectious HIV-1 particles. Because the HERV-K Gag is often truncated or mutated, these hybrid viral particles are immature, non-infectious, and exhibit significant defects in release, effectively reducing the viral load and the spread of the exogenous infection.
Innate Sensing and the Double-Edged Sword of dsRNA
A process called “viral mimicry” allows HERVs to proactively prime the immune system. When the epigenetic silencing of certain HERV loci is relaxed, they can be transcribed in both the forward and reverse directions, producing double-stranded RNA (dsRNA). This dsRNA is recognized by pattern recognition receptors (PRRs) like MDA5 and TLR3, which are designed to detect modern viral threats. This triggers a low-level, chronic activation of the innate immune response, which can be beneficial by keeping the immune system in a state of “ready alert,” but can also be detrimental if it leads to pathological inflammation.
Clinical Manifestations: Pathological Reactivation and Chronic Disease
While HERVs are essential contributors to human physiology, their dysregulation is a major factor in the progression of numerous complex diseases. When the robust epigenetic mechanisms that usually silence these elements—such as DNA methylation and H3K9me3—are disrupted by environmental triggers, aging, or infection, the resulting reactivation can be catastrophic.
Neuroinflammatory Cascades in Multiple Sclerosis and ALS
The abnormal expression of the HERV-W and HERV-K families has been heavily implicated in neurodegeneration. In Multiple Sclerosis (MS), the HERV-W envelope protein (MSRV-Env) has been detected within the active lesions of patients, where it acts as a potent agonist for Toll-like receptor 4 (TLR4) on microglia and macrophages. This interaction triggers a pro-inflammatory cascade that contributes directly to demyelination and axonal damage. Clinical trials are now exploring monoclonal antibodies designed to neutralize the HERV-W Env protein as a potential therapy for MS.
In Amyotrophic Lateral Sclerosis (ALS), there is significant evidence of HERV-K (HML-2) reactivation. High levels of HERV-K transcripts and proteins have been detected in the brain tissue and cerebrospinal fluid (CSF) of ALS patients. Transgenic animal models expressing the HERV-K env gene exhibit selective loss of motor neurons and progressive motor dysfunction, suggesting that the viral protein itself may be neurotoxic. The presence of anti-HERV-K antibodies in the blood of ALS patients further supports the idea of a systemic immune response to these re-emerging ancient viruses.
Alzheimer’s Disease and the Aging Genome
As the human genome ages, global DNA methylation levels tend to decrease, leading to the gradual derepression of retroviral elements. In Alzheimer’s Disease (AD), increased levels of HERV-K (HML-2) and HERV-W transcripts have been observed in post-mortem brain samples. Research has shown that HERV-K RNA can induce neurodegeneration through Toll-like receptor 8 (TLR8) signaling, suggesting that the accumulation of retroviral transcripts may be a driver of the chronic inflammation seen in AD.
Malignant Transformations: HERVs in Human Oncogenesis
Cancer cells frequently exhibit “genomic instability” and a profound loss of epigenetic control, creating a permissive environment for HERV activation. HERVs contribute to oncogenesis through several distinct mechanisms, from structural proteins that promote metastasis to regulatory elements that hijack the cell cycle.
Accessory Proteins: The Oncogenic Mechanics of Rec and Np9
The HERV-K (HML-2) family produces two accessory proteins, Rec and Np9, which are frequently detected in malignant tissues. Np9 acts as a molecular switch for several critical signaling pathways; it can activate the Notch, AKT, and ERK1/2 pathways, all of which are associated with cellular transformation and survival. Np9 has also been shown to promote the growth of leukemia stem cells and upregulate oncogenic factors like β-catenin.
The Rec protein interacts with tumor suppressors like PLZF (promyelocytic leukemia zinc finger), which normally represses the c−myc oncogene. By sequestering PLZF, Rec leads to the derepression of c−myc, fueling uncontrolled cell proliferation. Furthermore, Rec can interact with androgen receptors, potentially contributing to the progression of prostate and testicular cancers.
The table below outlines the prevalence of HERV expression across various tumor types.
| Tumor Type | Active HERVs Detected | Potential Mechanisms / Products |
| Breast Cancer | HERV-K, HERV-H, HERV-R, HERV-P | Env induces EMT and activates ERK pathway |
| Melanoma | HERV-K (HML-2) | Env-mediated cell-cell fusion; Rec protein expression |
| Prostate Cancer | HERV-K (HML-2), HERV-E | Gag and Env proteins; Transcriptional regulation |
| Ovarian Cancer | HERV-K, HERV-W, HERV-E, ERV3 | Env proteins; Hypomethylation of viral loci |
| Leukemia/Lymphoma | HERV-K (HML-2), HERV-W | Np9 signaling; LTR acting as alternative promoter |
| Lung Cancer | HERV-K, HERV-H, HERV-R, HERV-P | Env protein expression; Insertion polymorphisms |
LTR Transactivation and Tumor Immune Escape
HERV LTRs can also act as “hidden” promoters for host oncogenes. Because LTRs are repetitive and dispersed, they can occasionally integrate near critical cell-cycle regulators. In some lymphomas, for example, a MaLR LTR acts as an alternative promoter for the CSF1R oncogene, driving its overexpression.
Additionally, the over-expression of HERV-W env (Syncytin-1) in various cancers, including endometrial and urothelial carcinoma, is thought to assist in tumor immune escape. By mimicking the immunosuppressive and fusogenic properties required during pregnancy, cancer cells may create a protective “shield” that prevents T cells from recognizing and attacking the tumor.
The Frontier of Paleovirology: 2025 Research and Structural Biology
As of 2025, the scientific understanding of HERVs has transitioned from genomic surveys to high-resolution structural and proteomic analysis, allowing for the first time the precise targeting of these elements for clinical use.
The Gleason Integrated Approach: Resolving RNA-Protein Discordance
A landmark study published in Nucleic Acids Research in January 2025 addressed a critical gap in the field: the fact that high RNA expression from a HERV locus does not always mean a protein is being produced. By developing a proteogenomic pipeline that combined RNA-seq with mass spectrometry (MS) of membrane-enriched cell fractions, researchers identified the specific HML-2 loci that are the predominant source of viral proteins.
In NCCIT cells, the study identified 44 different HML-2 peptides, revealing that loci such as 1q22, 3q13.2, and 22q11.23 were actively translating Gag proteins. Notably, the 1q22 locus was found to have an intact Gag ORF despite previous genomic data suggesting it was truncated, highlighting the importance of cell-specific sequencing. This precision mapping allows researchers to identify which specific “active” viral remnants are the true culprits in disease, rather than relying on global transcriptional levels.
Structural Resolution of the HERV-K Env Glycoprotein
In September 2025, a team at the La Jolla Institute for Immunology achieved a milestone in structural biology by decoding the 3D structure of the HERV-K Env protein—the surface glycoprotein of the most active HERV family. This was only the third retroviral envelope structure ever solved, following HIV and SIV.
The structural data revealed that the HERV-K Env protein has a unique, “twitchy” shape that changes depending on its environment. This breakthrough is transformative for diagnostics and therapy. Because HERV-K Env is specifically displayed on the surface of many tumor cells and on neutrophils in patients with lupus and rheumatoid arthritis—but not on healthy cells—the resolved structure allows for the design of highly specific antibodies. These can be used to develop “smart” immunotherapies that kill cancer cells while sparing healthy tissue, or as diagnostic tools to identify autoimmune flare-ups with unprecedented accuracy.
Thematic Epilogue: The Symbiotic Self
The history of the human genome is a story of radical inclusion. The 8% of our DNA that is retroviral represents a legacy of nearly 100 million years of primate evolution, a record of every major infection that failed to kill us and instead became a part of us. We are the living evidence that pathogens and hosts can achieve a state of permanent, productive peace.
The “thousands of highly active” HERVs discussed here are the bridge between our viral past and our human present. They are not genomic baggage; they are the tools that allow our embryos to implant, our brains to think, and our immune systems to stand guard. This perspective shifts our understanding of biology from a struggle for purity to an appreciation of complexity. To be human is to be an integrated ecosystem, a host for the ghosts of ancient viruses that now provide the essential architecture for our existence.
As we move into a future of precision medicine, the targeting of HERVs will likely become a cornerstone of therapy for neurodegeneration and cancer. Yet, we must approach this with a nuanced understanding of the delicate balance that has been struck over eons. We cannot simply “cure” our genome of its viral components, for they are the very things that made us what we are. The viral architect resides within us, a silent partner in the ongoing evolution of the human species, reminding us that we are, and have always been, a collaborative work of nature.




Leave a comment