Presented by Zia H Shah MD
Audio teaser:
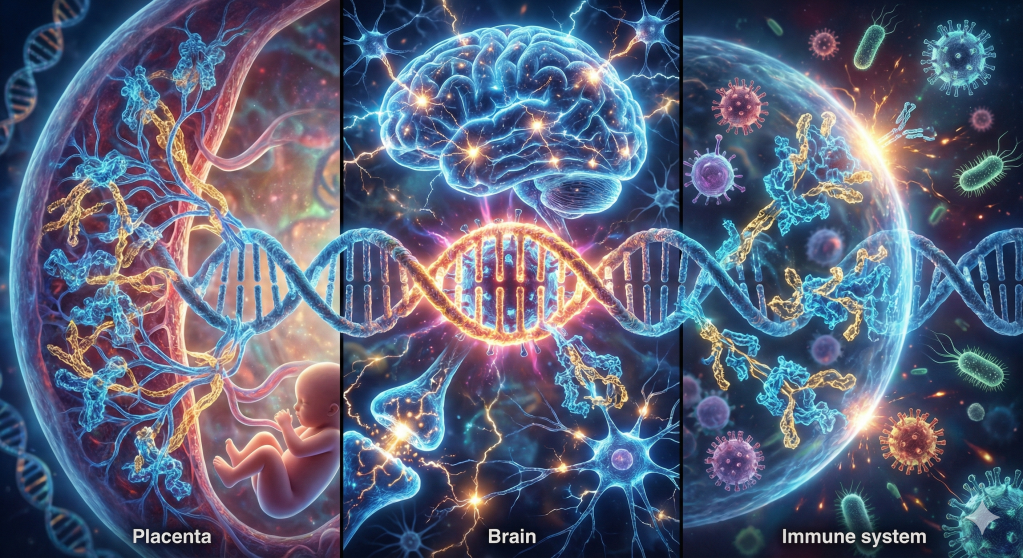
The traditional view of the human genome as a stable, purely mammalian sequence of instructions has been fundamentally dismantled by the emergence of paleovirology and high-resolution genomic mapping. Biological reality suggests that the human identity is not a solitary lineage but a complex, chimeric assembly. Approximately 8% of the human genetic code is composed of ancient viral DNA, remnants of ancestral infections that occurred millions of years ago. These sequences, known as human endogenous retroviruses (HERVs), represent the fossilized traces of exogenous retroviruses that successfully integrated into the germline of our ancestors, ensuring their vertical transmission across countless generations. Far from being inert “junk DNA,” these elements have been “usefully employed” for the most fundamental processes of human existence, including the development of the placenta, the regulation of the brain’s cognitive architecture, and the defense against modern pathogens. The transition of these elements from lethal parasites to essential symbiotic partners represents one of the most profound narratives in evolutionary history—a story not of blind happenstance, but of a sophisticated, integrated genomic ecosystem that points toward a guided and purposeful progression of life.
The Intentional Mosaic
The human genome is far more than a pristine blueprint; it is a palimpsest, a record of ancient battles and unlikely alliances where the distinction between “self” and “foreign” has been erased by a process that appears increasingly intentional. We are, in a very real biological sense, descendants of viruses as much as we are descendants of primates. The 100,000 fragments of retroviral DNA embedded in our chromosomes are not mere historical debris; they are active, vibrant participants in the daily business of life. This integrated genomic architecture challenges the reductionist view of “blind evolution,” where random mutations are the sole drivers of change. Instead, the emergence of vital functions from “junk” suggests a Master Designer or a Providential hand that embedded resilience and adaptability into the very fabric of creation. These viral “ghosts” are the engineers of our embryos, the sentinels of our immune systems, and the architects of our memories, serving as a wellspring of evolutionary innovation that defies the odds of random accident.
The Genomic Landscape: Origin and Architecture of Human Endogenous Retroviruses
The presence of human endogenous retroviruses is the result of a process known as endogenization. This occurs when an exogenous retrovirus infects a germline cell and its proviral DNA is successfully integrated into the host’s chromosomal DNA. Over millions of years, these sequences have undergone repeated rounds of amplification and transposition, leading to their widespread distribution throughout the genome.
Taxonomic Diversity and Classification Hierarchies
The human genome contains fragments categorized into roughly 30 to 40 distinct families. These are primarily grouped into three major classes:
- Class I: Similar to Gammaretroviruses (e.g., HERV-H, HERV-W).
- Class II: Related to Betaretroviruses; includes the highly active HERV-K superfamily.
- Class III: Related to Spumaviruses (e.g., HERV-L).
| HERV Family | Primary Location (Chromosome; Band) | tRNA Primer | Estimated Age (106 years) | Copy Number in Genome |
| HERV 1 | 10; q11.21 | Pro | 12.2 – 29.7 | ~21 |
| HERV 4 | 4; q13.3 | Glu | 24.2 – 62.2 | ~170 |
| HERV 11 | 1; p35.2 | ND | 19.3 – 30.1 | ~67 |
| HERV-K (HML-2) | Multiple | Lys (K) | 0.1 – 5.0 | ~91 proviruses |
| HERV-H | Multiple | His (H) | ~30.0 | ~1,000 elements |
The Non-Random Blueprint: Natural Genetic Engineering and Genomic Intelligence
The perspective of “guided evolution” is most strongly supported by the discovery that viral integration and re-purposing are not random events. Leading theorists now propose that evolution is a process of “Natural Genetic Engineering,” where cells act as intelligent agents capable of actively modifying their own DNA in response to environmental stressors.
Non-Random Integration and Site Preference
Contrary to the “blind” model, retroviral insertion is often site-specific. Some viruses preferentially insert near the start sites of active genes or specific regulatory regions, acting more like precision tools than random invaders. This non-randomness suggests that the genome possesses an inherent “molecular syntax” that recognizes and incorporates viral content at pre-planned sites to facilitate new functions.
“Virolution”: Viruses as Competent Editors
The theory of Virolution posits that viruses are not just pathogens but the primary agents of genetic innovation. Rather than relying on “beneficial errors,” evolution utilizes consortial swarms of viruses to edit host code, a process characterized as “Natural Genome Editing.” This biosemiotic approach views the genome as a highly formatted, “read-write” database constantly being updated through sophisticated, rule-governed interactions. In this light, the human species is the yield of “coordinate cellular problem-solving” rather than the accidental survivor of millions of random mutations.
Developmental Catalyst: HERVs in the Early Life Cycle
Specific retroviral elements are transiently and robustly activated to drive key developmental transitions, a mechanism that appears far too coordinated for an undirected process.
- HERV-L and Zygotic Activation: Indispensable for the 2-cell stage; without this viral-driven activation, the embryo cannot progress.
- HERV-H and Pluripotency: HERV-H transcripts function as regulatory scaffolds for core genes like OCT4 and NANOG. Knocking down these viral RNAs results in an immediate loss of stemness.
- Syncytins and the Placenta: The fusion of trophoblast cells to form the placenta is mediated by captured viral envelope proteins, Syncytin-1 and Syncytin-2. This “viral exaptation” allowed for the evolution of live birth, utilizing an invader’s tools to ensure a symbiotic barrier between mother and fetus.
The Cognitive Virus: HERVs in Neurobiology and Human Thought
The integration of retroviral elements into the brain has provided the hardware for memory and possibly the catalysts for the expansion of the human mind.
The Arc Gene: Viral Hardware for Memory
The Arc gene, essential for long-term memory, is derived from an ancient retrotransposon. Arc protein self-assembles into hollow capsids that package mRNA and travel between neurons, a process resembling a “domesticated infection” used for intercellular communication.
mTOR Activation and Brain Expansion
Recent 2025 research suggests that HERV-K activation triggers the mTOR pathway in developing brain cells, a pattern that specifically distinguishes humans from other primates. This viral “genetic switch” may have ramped up neural growth, enabling the blossoming of human cognitive capacity. From a guided perspective, this represents a providential “tweak” that allowed for the emergence of human consciousness and language.
Immunological Sentinels: Viral Mimicry and Host Defense
The “domestication” of viral activities has turned ancient invaders into the vanguard of our immune system.
- MER41 and Interferon: Thousands of HERV-derived LTRs (specifically the MER41 family) act as enhancers to boost the expression of antiviral genes when the immune system detects an infection.
- Super-infection Resistance: HERV proteins can block the receptors used by modern viruses or disrupt their assembly, providing a form of “genetic vaccination” against modern threats.
Clinical Manifestations and 2025 Research
While essential, the dysregulation of these elements is linked to neurodegeneration (MS, ALS) and cancer. However, 2025 breakthroughs have provided new tools for control:
- Structural Resolution: Solving the 3D structure of the HERV-K Env protein has opened doors to “smart” immunotherapies that target cancer cells displaying this viral signature while sparing healthy tissue.
- Precision Mapping: Proteogenomic pipelines in 2025 now distinguish which specific HERV loci (such as 1q22 and 22q11.23) are translating proteins, allowing for targeted diagnostic interventions.
Thematic Epilogue: The Collaborative Masterpiece
The history of the human genome is not a chronicle of accidents, but a story of radical and purposeful inclusion. The 8% of our DNA that is retroviral represents a legacy of nearly 100 million years of primate evolution—a record of every major infection that failed to kill us and was instead transformed into a vital component of our being. We are the living evidence that pathogens and hosts can achieve a state of permanent, productive peace.
This evidence of “Natural Genetic Engineering” and the re-purposing of viral “junk” into the foundations of the placenta, the brain, and the immune system strongly reinforces the perspective of guided evolution. It suggests that life is not a solitary struggle against a hostile environment, but a collaborative masterpiece built upon the captured genetic hardware of ancient travelers. The “thousand active sites” in our genome are not baggage; they are the tools of a “Viral Logos”—an inherent logic within the genome that utilizes the most unlikely materials to build complexity.
To be human is to be an integrated ecosystem, a host for the ghosts of ancient viruses that now provide the essential architecture for our thought and our survival. The viral architect resides within us, a silent partner in our ongoing creation, reminding us that we have never been “blind” products of chance, but rather the intended outcome of a sophisticated and deeply cooperative biological plan.




Leave a comment